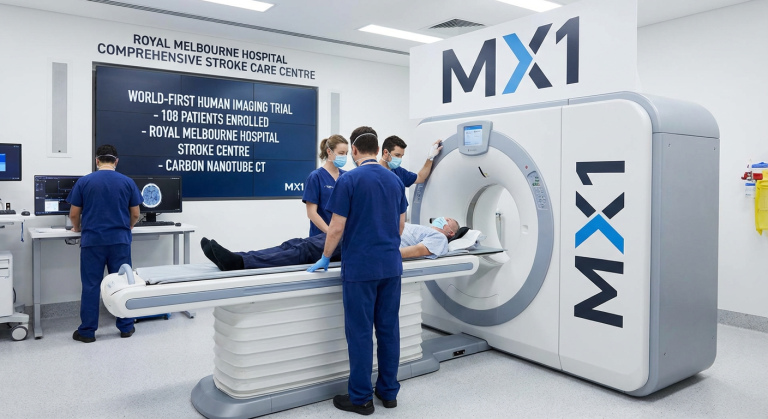
Micro-X Limited (ASX: MX1) has activated its first hospital trial site for the Micro-X Head CT stroke trial at Royal Melbourne Hospital, marking the transition from laboratory development to world-first human imaging using carbon nanotube CT technology. The activation at RMH, a globally recognised Comprehensive Stroke Care Centre and home to the Melbourne Brain Centre (the largest brain research collaboration in the southern hemisphere), represents a critical step toward regulatory submissions and commercial validation of the company’s portable stroke diagnostic platform.
Installation and commissioning of the Head CT test bench commenced this week at RMH, with final technical testing and staff training underway. Before human imaging begins, a formal phantom study will be completed with evaluation by neuroradiologists and vascular neurologists, alongside comprehensive dosimetric analysis. The structured approach reflects the company’s focus on data quality and technical excellence as it progresses toward clinical validation.
The multi-centre prospective pilot study will enrol 108 patients across an estimated 6-month period. Patients will include those presenting with ischaemic stroke, intracranial haemorrhage, brain tumour, or stroke mimic conditions. The study’s primary objective centres on investigating the scanner’s ability to identify neuroimaging-based contraindications to stroke thrombolysis, a time-critical clinical decision that directly impacts patient outcomes.
Academic partner Australian Stroke Alliance brings together more than 40 organisations committed to improving pre-hospital stroke care in Australia, providing clinical governance and research infrastructure for the trial. This collaboration positions the technology within a nationally coordinated stroke care framework.
COO Anthony Skeats on carbon nanotube CT advancement
“The activation of our first trial site is a major step towards world-first human imaging using carbon nanotube CT developed by Micro-X. Our CT technology has the potential to cut crucial time to stroke diagnosis and the commencement of treatment by bringing miniaturised CT to the patient. As we move through the final stages before the planned commencement of human imaging, we are maintaining our disciplined approach with a focus on data quality and technical excellence.”
For investors, this activation represents progression from development phase into clinical validation. Successful human imaging at a premier stroke centre would substantially de-risk the regulatory pathway ahead of formal submissions to health authorities.
Understanding portable CT technology for stroke diagnosis
Stroke treatment operates within an exceptionally narrow therapeutic window. Current clinical protocols typically require patient transport to hospital-based CT facilities before treatment can commence, introducing delays that directly correlate with worse patient outcomes. Portable CT technology addresses this constraint by enabling diagnosis at the point of care, whether in emergency departments or potentially in ambulances.
Micro-X’s cone beam brain scanner employs cold cathode carbon nanotube emitter technology, a fundamentally different architecture compared to conventional CT systems. Traditional CT scanners use heated filament technology, requiring substantial power infrastructure and occupying room-sized installations. The carbon nanotube approach enables electronic control of the X-ray emitter, permitting significant reduction in size, weight and power requirements. This miniaturisation creates the technical foundation for mobile deployment scenarios previously impossible with conventional technology.
The clinical investigation follows a structured design pathway:
- Four patient categories: Ischaemic stroke, intracranial haemorrhage, brain tumour, and stroke mimic presentations
- Primary objective: Identifying contraindications to stroke thrombolysis using the portable scanner
- Study design: Prospective multi-centre pilot with within-participant comparison against standard imaging protocols
This design generates comparative data essential for regulatory assessment, directly evaluating whether the portable system can deliver clinically equivalent diagnostic information to existing hospital-based CT infrastructure.
Trial design and pathway to regulatory applications
The phased approach to human imaging reflects medical device development requirements. Prior to enrolling patients, the phantom study will establish baseline imaging performance characteristics and radiation dosimetry under controlled conditions. Formal evaluation by specialist neuroradiologists and vascular neurologists provides independent clinical assessment before progression to human subjects.
Data generated through the pilot study is designed to support future regulatory submissions for the Head CT scanner. The study protocol’s within-participant comparison design (where each patient receives both the investigational scan and standard-of-care imaging) provides the comparative effectiveness data typically required by regulators assessing new diagnostic technologies.
Parallel to hospital-based trials, Micro-X is advancing development of the first in-ambulance Head CT prototype under Australian Government Industry Growth Program funding. Two additional test benches are in advanced construction at the company’s Adelaide manufacturing facility, enabling second and third trial sites to commence imaging following RMH’s lead. The first ambulance prototype milestone, covering planning and system architecture, has been submitted to the funding body.
This parallel development strategy compresses the overall timeline from hospital validation to pre-hospital deployment. Hospital trial data informs ambulance prototype specifications, whilst prototype engineering proceeds concurrently rather than sequentially. For investors, this approach demonstrates efficient capital deployment and reduces the risk of lengthy sequential development phases.
The company’s vertically integrated Adelaide manufacturing facility provides control over production processes and component supply chains, potentially accelerating iteration cycles as clinical feedback is incorporated into system refinement.
Strategic partnerships accelerating clinical validation
The institutional credibility of trial partners reinforces the technology’s clinical potential. Royal Melbourne Hospital operates as an internationally recognised leader in stroke research and care, providing not just a trial site but specialist clinical expertise in stroke imaging assessment. The Melbourne Brain Centre collaboration brings together research infrastructure and multidisciplinary expertise across neurology, radiology and clinical trials.
Australian Stroke Alliance’s involvement extends beyond academic partnership. With more than 40 national organisations participating, the alliance represents coordinated stroke care infrastructure across Australia. This network provides both clinical trial capabilities and potential future adoption pathways if the technology receives regulatory approval.
Government support through the Medical Research Future Fund Frontiers programme validates the technology thesis from a public health policy perspective. Frontiers funding specifically targets technologies with potential to address significant unmet clinical needs. For a portable stroke CT platform, this aligns with documented evidence that delays in stroke diagnosis directly impact patient survival and functional outcomes.
The trial team comprises experienced neurologists, radiographers and radiologists, ensuring specialist oversight of imaging protocol development and data interpretation. This clinical governance structure supports the generation of high-quality evidence suitable for regulatory review.
Dr Anna Balabanski on Australian innovation pathway
“This is a significant milestone in the development of this Australian-made portable CT brain scanner. The Royal Melbourne Hospital is an international leader in advanced stroke care. This trial, led by experienced neurologists, radiographers, and radiologists, is a critical step towards enabling safe, high-quality brain imaging in the pre-hospital setting, once these in-hospital trials have demonstrated the device’s performance.”
| Trial Element | Detail |
|---|---|
| Sample Size | 108 patients |
| Enrolment Duration | ~6 months |
| Sites | 3 hospital locations |
| Clinical Partner | Australian Stroke Alliance |
| Funding Support | Medical Research Future Fund Frontiers |
Upcoming catalysts and development timeline
The 6-month enrolment period establishes a clear visibility window for investors monitoring clinical progress. Near-term milestones include completion of the phantom study at RMH, commencement of human imaging, and activation of second and third hospital sites as additional test benches complete construction and installation.
Parallel ambulance prototype development provides additional catalyst opportunities. Following submission of the first milestone (planning and system architecture), subsequent milestones will likely address prototype construction, component integration and initial performance testing. These milestones occur independently of hospital trial progress, creating multiple potential value inflection points.
Key upcoming milestones investors should monitor:
- Completion of phantom study and neuroradiologist evaluation at RMH
- Commencement of patient enrolment and human imaging
- Activation of second and third hospital trial sites
- Ambulance prototype construction milestones under Industry Growth Program funding
- Interim data readouts (timing dependent on enrolment rate and protocol design)
The structured approach from phantom study through human imaging reflects the conservative, data-driven methodology appropriate for medical device development. Whilst this creates a longer pathway to commercialisation compared to less regulated industries, it also systematically addresses regulatory requirements and clinical validation in sequence.
Investment thesis: de-risking the path to commercialisation
The RMH activation strengthens the investment case by demonstrating tangible progress from development phase into clinical validation. Medical device companies face substantial regulatory and clinical risk until human data confirms that laboratory performance translates into real-world clinical utility. Successful imaging at world-leading stroke centres would represent material de-risking ahead of regulatory submissions.
The technology addresses a documented clinical need where delays in diagnosis directly impact patient outcomes. Portable CT capable of delivering hospital-quality imaging at the point of care could compress the critical time window between symptom onset and treatment initiation. This clinical value proposition underpins the commercial opportunity, as healthcare systems globally prioritise technologies that improve patient outcomes whilst potentially reducing costs associated with delayed treatment.
Micro-X’s vertically integrated manufacturing capability in Adelaide provides production control and supply chain independence, important factors for scaling from clinical trials into commercial production. The company’s expanding US presence through its Seattle technical and commercial team positions it for the large North American market opportunity, whilst hospital trials in Australia generate the clinical evidence required for regulatory submissions in multiple jurisdictions.
Multiple parallel development tracks (hospital trials, ambulance prototype, US expansion) demonstrate efficient capital deployment and create diverse potential value inflection points over the coming 12-18 months. The company’s broader product portfolio spanning healthcare and security applications (including contracts with US Department of Homeland Security and ARPA-H) provides business diversification beyond the stroke imaging platform.
Successful completion of the pilot study would position Micro-X to pursue regulatory approvals and advance toward commercial deployment, potentially unlocking significant enterprise value for a technology addressing a global clinical need.
Don’t Miss the Next MedTech Breakthrough
Join 20,000+ investors receiving FREE breaking ASX healthcare news within minutes of release, complete with in-depth analysis. Click the “Free Alerts” button at StockWire X to get market-moving announcements and expert coverage delivered straight to your inbox before the broader market reacts.