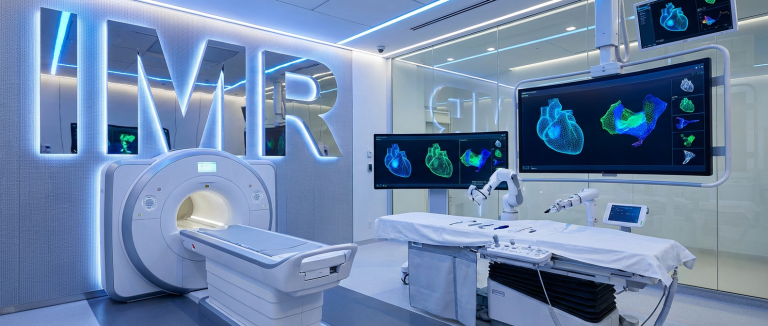
Imricor Medical Systems (ASX: IMR) has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its NorthStar Mapping System, marking the company’s second FDA clearance in January 2025. The Imricor NorthStar FDA clearance positions the medical device manufacturer as the only company with an MRI-native 3D mapping and guidance system approved for the US market, unlocking access to the world’s largest electrophysiology market.
This regulatory milestone follows the company’s earlier 510(k) clearance for the VisionMR Diagnostic Catheter, demonstrating rapid execution on its US market entry strategy. NorthStar represents Imricor’s first capital equipment and software-centric product approval in the United States, establishing a foundational platform for the company’s expanding pipeline of MRI-guided interventional products.
Key highlights from the announcement:
- First and only: NorthStar is the first MRI-native 3D mapping and guidance system to receive FDA clearance
- Capital equipment milestone: First capital equipment and software-centric product approval for Imricor in the US
- Platform foundation: Designed as the foundational software guidance system for existing and future MRI-guided procedures
What is the NorthStar Mapping System?
The NorthStar Mapping System is designed to serve as the central hub for interventional cardiac MRI (iCMR) laboratories. The system provides real-time 3D mapping and guidance capabilities for cardiac procedures performed under MRI guidance, enabling clinicians to conduct diagnostic electrophysiology studies and ablation procedures in a completely radiation-free environment.
Unlike traditional x-ray fluoroscopy-guided procedures, NorthStar harnesses MRI’s superior soft tissue imaging capabilities to precisely guide minimally invasive cardiac interventions. The system is intended to support not only current diagnostic and ablation procedures but also to provide guidance for future MRI-based interventions as the company’s product portfolio expands.
NorthStar’s core capabilities include:
- 3D cardiac mapping: Real-time three-dimensional visualisation of cardiac anatomy and electrical activity
- MRI guidance integration: Seamless integration with MRI imaging for procedure navigation
- Radiation-free environment: Elimination of ionising radiation exposure for patients and clinical staff
- Expandable platform: Software architecture designed for continuous capability enhancement
Why software matters for Imricor’s future
The Imricor NorthStar FDA clearance signals a strategic shift for the company as it enters what management describes as its “software era.” According to CEO Steve Wedan, the platform has been in development for two decades, and its software-centric architecture creates opportunities for virtually unlimited capability expansion.
This transition to software products carries significant commercial implications. Software-based medical devices typically command higher margins than hardware-only products and create opportunities for recurring revenue through upgrades and enhanced functionality. Management has indicated that artificial intelligence integration will play a substantial role in NorthStar’s future development, potentially adding diagnostic support and procedural automation capabilities over time.
The platform architecture allows Imricor to introduce new features and capabilities without requiring hardware replacement, creating a pathway for sustained value delivery to installed customer base sites.
US market access and commercial runway
FDA clearance enables Imricor to immediately commence commercial marketing of the NorthStar Mapping System in the United States, the world’s largest electrophysiology market. This regulatory approval transforms the company’s addressable market, shifting from primarily European operations to access across the world’s most valuable healthcare market.
Management expects to secure multiple additional regulatory clearances and approvals throughout 2025, progressively introducing Imricor’s complete MRI-guided electrophysiology platform to US clinicians. The company’s products currently hold approvals across several international markets, with the US representing the most commercially significant expansion to date.
| Geography | Regulatory Status |
|---|---|
| United States | Two 510(k) clearances (January 2025) |
| European Union | Approved |
| Kingdom of Saudi Arabia | Approved |
| New Zealand | Approved |
| Australia | Planned |
The staged regulatory approach allows Imricor to build a comprehensive product suite in the US market, with each clearance enabling additional procedural capabilities and revenue opportunities. The company is progressively introducing its full platform rather than seeking single omnibus approval, potentially accelerating time to market for individual products.
Management commentary on strategic positioning
Executive Chair, President and CEO Steve Wedan positioned the NorthStar clearance within the company’s broader two-decade development programme, emphasising the system’s role in enabling better clinical outcomes at lower cost.
CEO Vision Statement
“At Imricor, we have been building a comprehensive suite of uniquely MRI-compatible devices for two decades. These devices, which include both consumable products and capital equipment, enable doctors to harness the superior soft tissue imaging of MRI to precisely guide minimally invasive procedures in a 100% radiation-free setting. Our goal is to enable better, faster, safer and less expensive treatments for patients worldwide; and as the world’s largest market, the United States is critical to our goal.”
Wedan further emphasised NorthStar’s central importance to the company’s product ecosystem and its software-driven future.
Platform Potential
“When it comes to iCMR procedures, NorthStar is the central hub that brings everything together. It’s designed to not only facilitate diagnostic cardiac electrophysiology and ablations procedures, but also to provide MRI guidance capabilities for other procedures. And since NorthStar is primarily a software product, it ushers in Imricor’s software era in which AI will play a big role in the future. NorthStar’s platform provides a path for capability expansion that is virtually unlimited, and we will continue to invest in and expand its capabilities for years to come.”
Building the iCMR ecosystem
Imricor’s product portfolio spans both capital equipment and single-use consumable devices, creating a commercial model where capital equipment placements drive ongoing consumable revenue. The company manufactures ablation catheters, diagnostic catheters, steerable sheaths, and supporting tools used in cardiac ablation procedures, all designed for compatibility with MRI guidance.
Imricor’s product categories include:
- Capital equipment: NorthStar Mapping System, Advantage-MR EP Recorder/Stimulator
- Single-use consumables: Ablation catheters, diagnostic catheters, steerable sheaths, procedural accessories
Each NorthStar installation creates a customer site that requires compatible consumable products for procedures, establishing recurring revenue relationships. As the only FDA-cleared MRI-native mapping system, NorthStar installations position Imricor as the exclusive supplier for MRI-guided cardiac procedures at those sites.
The system is designed to support not only current cardiac electrophysiology procedures but also to provide guidance capabilities for future MRI-based interventions as clinical techniques and product capabilities expand.
What this means for investors
The Imricor NorthStar FDA clearance validates the company’s technology platform and regulatory strategy whilst opening commercial access to the world’s most valuable electrophysiology market. Securing two 510(k) clearances in a single month demonstrates execution capability and suggests the company has established efficient regulatory processes that may accelerate future product approvals.
The shift to software-centric products carries margin implications, as software typically commands higher gross margins than hardware-only devices whilst creating opportunities for recurring upgrade revenue. Management’s emphasis on artificial intelligence integration suggests future capability expansions that could drive additional value from the installed base.
With multiple additional clearances expected throughout 2025, (ASX: IMR) is positioned to maintain consistent newsflow as additional products gain US approval. Each clearance expands the range of procedures that can be performed using Imricor’s MRI-guided platform, broadening the revenue opportunity from capital equipment installations.
The regulatory momentum positions Imricor to capture market share in MRI-guided interventional procedures as hospitals invest in radiation-free alternatives to traditional fluoroscopy-guided cardiac procedures. As the sole provider of FDA-cleared MRI-native mapping systems, the company holds a defensible competitive position in this emerging procedural category.
Want more Biotech breakthroughs delivered to your inbox?
The Imricor NorthStar FDA clearance demonstrates how rapidly regulatory catalysts can transform a biotech company’s commercial trajectory. For investors tracking ASX Biotech and Healthcare opportunities, staying ahead of market-moving approvals, clinical trial results, and strategic developments is essential. The Big News Blasts service delivers FREE breaking news and comprehensive analysis on exactly these types of catalysts across non-resource ASX sectors including Biotech, Healthcare, Tech, and Finance—trusted by 20,000+ active subscribers who demand timely, actionable intelligence.
To ensure no critical regulatory milestone, partnership announcement, or funding event passes unnoticed, subscribers can access instant alerts the moment news breaks. Readers interested in systematic coverage of ASX Biotech and Healthcare developments can join the community by clicking the “Free Alerts” button at StockWire X to receive curated analysis on the companies and sectors shaping Australia’s innovation economy.